New Type of Gene Engineering Is Aimed at Sidestepping Critics
By BARNABY J. FEDER
New York Times
March 9th, 2000
www.nytimes.com
Researchers estimate that rice and corn began evolving their separate ways from a common grassy ancestor at least 60 million years ago. But how far did they travel?
Intriguingly, researchers have found that the genes in corn that tell the plant what proteins to make to produce its shape, its cob, its roots and its reproductive system -- in short, everything that makes it corn -- have largely identical counterparts in rice, arrayed in pretty much the same order.
That suggests that the differences between the plants might come in large part not from the genes themselves, but from the point at which they are switched on and off, how strongly and in which part of the plant they are active. And now some wonder if this insight can be used for a new kind of genetic engineering.
No one is more enthusiastic about such inquiries than Dr. Richard A. Jefferson, the 43-year-old founder of the Center for the Application of Molecular Biology to International Agriculture in Canberra, Australia, who sometimes declares, "Rice is corn." Although he knows that is an exaggeration, it serves as a catchy introduction to how his institute is trying to reshape the tangled global debate over the role of genetic engineering in agricultural biotechnology.
Dr. Jefferson argues that the high degree of genetic overlap between the plants -- indeed, among all living things -- suggests that much of the gene swapping among species that has stirred up so much opposition to genetic engineering may be unnecessary. Perhaps, instead of moving a valuable trait like resistance to cold from a fish to a plant, genetic engineers could achieve the same result by goading the plant into a mutation that activates genes for cold tolerance already present in its DNA.
Dr. Jefferson, a native of Berkeley, Calif., is not opposed to moving genes among different forms of life. In fact, he first made his mark in biotechnology at the University of Colorado in 1985 by inventing a way to track the location and activity of genes as researchers moved them from one species to another. In 1987, in one of the first field tests of a genetically altered food crop, he raised genetically altered potatoes at the Plant Breeding Institute in Cambridge, England.
Today, though, Dr. Jefferson's nonprofit research center, known as Cambia, is pouring resources into a project to jumble and rejumble the on-off patterns of rice genes, hoping to unleash traits buried in rice that evolution might not get around to exhibiting for millions of years, if ever.
Promising mutants -- say rice that produces vitamin A in the grain -- could be developed into viable crops in developing countries within four or five years by crossing them with existing crops, according to Dr. Andrzej Kilian, who was hired to run the research. And Cambia is working with partners to apply the same concepts to cassava, cowpea and other plants vital to food supplies in developing countries.
"Evolution uses random forces all the time," Dr. Kilian said. "We are trying to speed it up and make it manageable."
Laboratory-driven mutation work is just one of many Cambia projects aimed at the needs of farmers in developing countries. Dr. Jefferson hopes such efforts will create a way for small businesses and developing countries to exploit biotechnology while skirting the fortress of patents that Monsanto, DuPont and other multinational giants have assembled.
And that, in turn, could please some current critics of biotechnology.
"It's a noble effort," said Hope Shand, research director of the Rural Advancement Foundation International U.S.A., a group based in Pittsford, N.C., which has been a high-profile opponent of the biotechnology industry. "Our main concern has always been who benefits and who controls the technology."
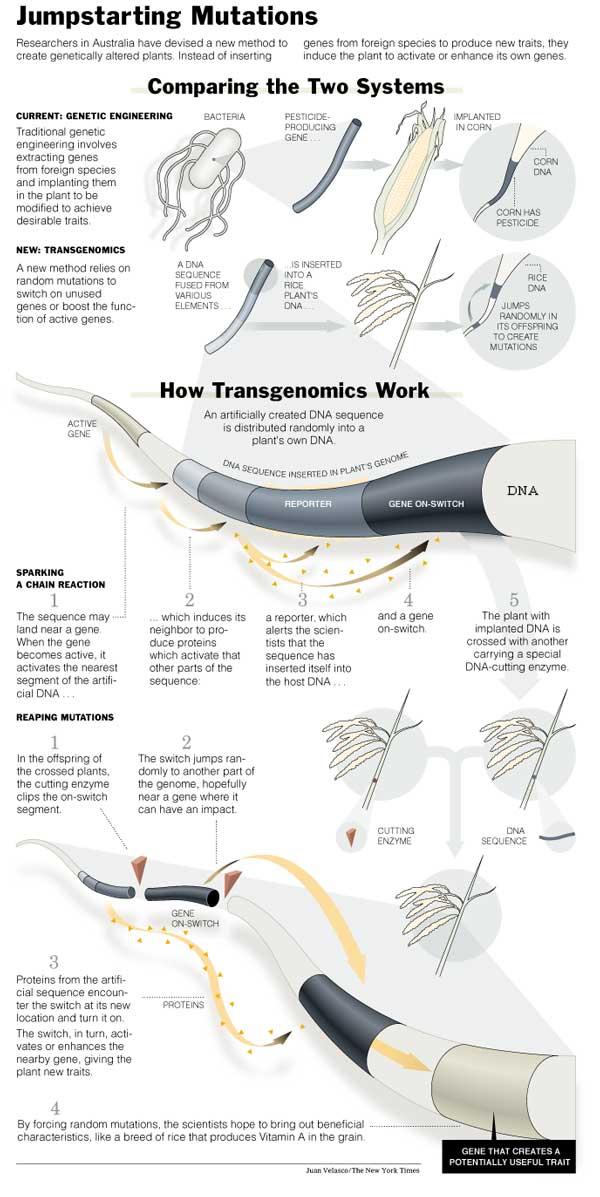
Cambia's technology still crosses species lines in ways that could upset some critics of biotechnology. Cambia's process, which it calls transgenomics, does not move genes intended to introduce a novel trait into rice, but its method for changing rice's regulation of its own genes does require on-off switches to be imported from bacteria, yeast or other plants.
"People may look at this differently than some of the transspecies work but some of the risks they worry about would still be there," said Dr. Margaret Mellon, who tracks biotechnology issues at the Union of Concerned Scientists in Washington.
Dr. Mellon said that Cambia's work might provide valuable research on plant genetics, but that those concerned with world hunger ought to see it as a poor alternative to breeding programs crossing rice with wild relatives, like that being run in China by Chinese researchers and Dr. Susan McCouch, a Cornell scientist.
Dr. McCouch has reported increasing yields on commercial rice strains by 10 percent to 20 percent by crossing them with wild species. In one case, the offspring surprisingly proved to be resistant to a virus plaguing Latin American rice growers, even though neither parent was.
Such work is often cited by opponents of biotechnology as proof that genetic engineering is unnecessary and distracting, but Dr. McCouch herself doubts that traditional breeding alone will meet the developing world's food needs. Dr.
McCouch and others say the transgenomics program is a logical, if bold, use of the growing mountain of research on how mutations occur naturally.
It has been known for decades that organisms occasionally cut loose pieces of their own regulatory DNA so that they can jump, apparently randomly, to other locations and possibly activate genes with helpful traits. Production of the enzymes, or transposases, that free the mobile DNA, or transposons, increases when organisms are under almost any kind of stress.
"One of the genome's last ditch responses under stress is to reshuffle the deck," Dr. McCouch said.
Cambia is trying to mimic the natural process by inserting a packet of DNA from yeast, bacteria and corn DNA that can only be activated when a rice gene is nearby and active. When the rice gene is switched on, it simultaneously turns on a cascade of activity in the inserted DNA packet: one result is that a "reporter" gene -- Cambia's comes from bacteria -- begins producing a protein that has no effect on the plant but can be used by researchers to figure out where the packet has landed in the rice genome and how powerfully the nearby rice gene is working.
The activity also turns on DNA fragments that Cambia takes from yeast that amplify gene activity. That can boost the activity of the adjacent rice gene, which may produce noticeable changes in the plant. But Cambia's real mutation thrust is based on including in the DNA packet a DNA package that can become mobile when the plant is crossed with another plant containing an enzyme that frees it.
If the mobile segment jumps from, for example, a location next to a rice gene active during seed development to the neighborhood of a gene involved in root growth, it will be turned on and stimulate the root gene when the seed gene becomes active.
That process mimics what a rice transposon might do naturally if the plant were under stress. Researchers must still screen thousands of plants, just as natural breeders do, looking for mutations that are beneficial to farmers.
Cambia is now building a library of plants with the DNA packages inserted near various rice genes. It has about 3,000 rice plants and expects to reach a goal of 10,000 by the end of the year.
Theoretically, there is plenty of room to create new functions for existing genes. "Plant genes are generally redundant," said Dr. Susan Wessler, an expert in corn and rice genetics at the University of Georgia. "There may be eight copies of a gene." Thus, one copy of the gene could be reregulated to produce a new trait by a transposon without necessarily compromising the plant.
Multinational companies have been notably silent about whether they are doing similar research. Dr. Jefferson said some were negotiating nonexclusive licensing agreements for transgenomics technology, parts of which could be used to make existing genetic engineering more precise.
For instance, corn might be engineered so that its bacterially derived pesticide gene is turned on only in the parts of the plant that corn borers eat. Now, engineered corn expresses the pesticide all over the plant, including in pollen that can be deadly to monarch butterflies and other beneficial insects.
But plenty of admirers working in research are hoping Dr. Jefferson's aim of using transgenomics to perform an end run around the multinationals' current approach to biotechnology pays off.
"Richard's focus is on those people for whom agriculture is most vital," said Dr. Jeffrey Bennetzen, a Purdue University plant genetics researcher. "Our biggest problem is overproduction, but the developing world really needs crop improvement."